INFRA-STRUCTURE
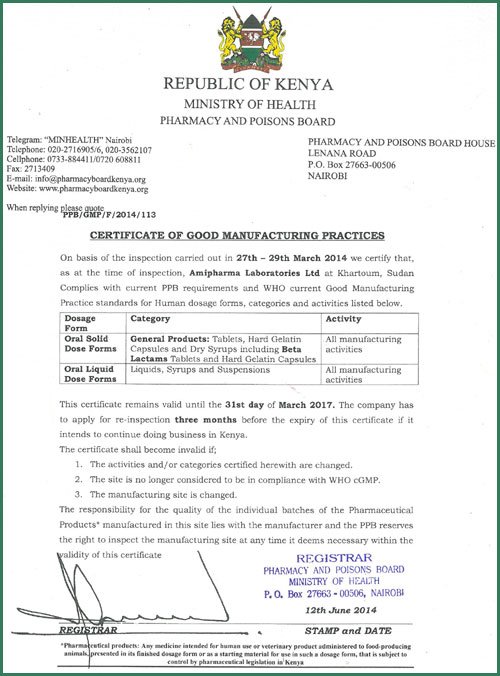
Our manufacturing facilities, quality control, storage and ancillary facilities were designed and executed in strict compliance with GMP (Good Manufacturing Practice) according to the latest WHO guidelines.
Our Quality control and Product Development Lab are well equipped with sophisticated equipment of high standards, precision and high technical knowhow and in strict compliance with the latest rules and regulations of Current Good Manufacturing Practices (cGMP) and Good Laboratory Practice.
COMPETITIVE ADVANTAGE
Throughout its 30 years of operation, Amipharma acquired its competitive position as the leading Sudanese pharmaceutical manufacturer based on its commitment to provide medicines that are safe, effective and at affordable prices, using state of the art facilities, latest machines and equipment, well trained human resource and its strong commitment to country development without compromising quality, business ethics and environmental requirements. The manufacturing areas are built and continuously rehabilitated to cope with the continuously changing requirements of the international regulatory standards.